
See a table of bond lengths and bond energies For the H 2 molecule, the distance is 74 pm (picometers, 10 -12 meters). Likewise, the difference in potential energy between the lowest energy state (at the optimal internuclear distance) and the state where the two atoms are completely separated is called the bond dissociation energy, or, more simply, bond strength. For the hydrogen molecule, the H-H bond strength is equal to about 435 kJ/mol.Įvery covalent bond in a given molecule has a characteristic length and strength. In general, the length of a typical carbon-carbon single bond in an organic molecule is about 150 pm, while carbon-carbon double bonds are about 130 pm, carbon-oxygen double bonds are about 120 pm, and carbon-hydrogen bonds are in the range of 100 to 110 pm. The strength of covalent bonds in organic molecules ranges from about 234 kJ/mol for a carbon-iodine bond (in thyroid hormone, for example), about 410 kJ/mole for a typical carbon-hydrogen bond, and up to over 800 kJ/mole for a carbon-carbon triple bond. There is a defined optimal distance between the nuclei in which the potential energy is at a minimum, meaning that the combined attractive and repulsive forces add up to the greatest overall attractive force. This optimal internuclear distance is the bond length. When the two nuclei are ‘too close’, we have an unstable, high-energy situation.

Overlap is above and below the bond axis, not directly between the bonded atoms.\)Īt first this repulsion is more than offset by the attraction between nuclei and electrons, but at a certain point, as the nuclei get even closer, the repulsive forces begin to overcome the attractive forces, and the potential energy of the system rises quickly.

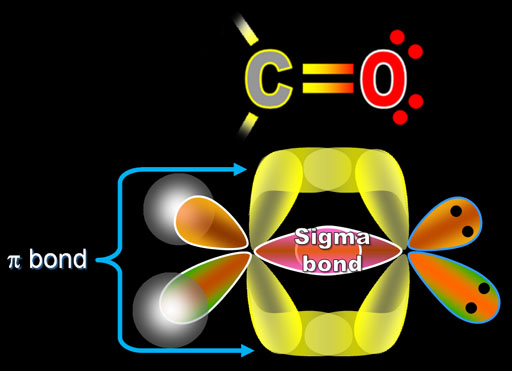
energy of isolated p orbitals bond order of a pi bond = (2)-(0) 2 = 1 bond 2p b π * = 2p a-2p b = antibonding MO = LCAO = linear combination of atomic orbitals ∆E = bond energy There is a big energy advantage for a pi bond over two isolated p orbitals. Problem 1 – What would the MO pictures of He 2, H 2 +, H 2-and He 2 + look like? Would you expect that these species could exist? What would be their bond orders? node = zero electron density because of opposite phases 2p a π bond LUMO HOMO π = 2p a + 2p b = bonding MO = potential energy higher, less stable lower, more stable LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively. There is a node between the bonding atoms (zero electron density). Sigma star (σ*) antibonding molecular orbital – Normally this orbital is empty, but if it should be occupied, the wave nature of electron density (when present) is out of phase (destructive interference) and canceling in nature. A sigma bonds is always the first bond formed between two atoms. Sigma (σ) bonding molecular orbital-Shared electron density is directly between the bonding atoms, along the bonding axis. energy of isolated atoms bond order (H 2 molecule) = (2)-(0) 2 = 1 bond 1s b H H H H H H σ * = 1s a-1s b = antibonding MO = LCAO = linear combination of atomic orbitals node = zero electron density because of opposite phases ∆E = bond energy There is a big energy advantage for a hydrogen molecule over two hydrogen atoms. Bond order = (number of bonding electrons)-(number of antibonding electrons) 2 = amount of bonding 1s a hydrogen molecule = H 2 LUMO HOMO σ = 1s a + 1s b = bonding MO = potential energy higher, less stable lower, more stable LUMO = lowest unoccupied molecular orbital HOMO = highest occupied molecular orbital Similar phase of electron density (no node) adds together constructively.


 0 kommentar(er)
0 kommentar(er)
